Introduction
Materials & Methods
1. Materials
2. Analytical methods
3. Adsorption studies of NH3-N and Na2S using SCGs
4. Adsorption kinetic and isotherm models of NH3-N and Na2S using SCGs
5. DNA extraction and next generation sequencing (NGS)
Results & Discussion
1. Influence of SCGs in cattle manure properties
2. Analysis of efficiency of removing odor-causing substances (NH3-N and Na2S) from SCGs
3. Adsorption kinetics and isotherms of NH3-N and Na2S using SCGs
4. Characterization of SCGs: pH and FT-IR analysis
5. Inoculation of a microorganism for the reduction of the odor of cattle manure
Conclusion
Introduction
South Korea is known worldwide for its high import and consumption of coffee beans, which has resulted in a significant increase in coffee by-products, particularly discarded coffee grounds (Tan et al., 2021). It is thus important to conduct research to increase the value of coffee grounds and promote environmentally friendly applications by recycling them as a waste resource. Spent coffee grounds (SCGs) are a by-product of coffee extraction and are known to contain organic matter, fiber, lignin, and caffeine (Hoseini et al., 2021). Recent studies have shown that SCG and SCG-derived activated carbon can be used to mitigate pollutants such as cadmium, tetracycline, ozone, and CO2 (Liu et al., 2018; Dai et al., 2019; Hsieh and Wen, 2020; Kim and Kim, 2020). Previous studies have also reported that SCGs are effective in reducing odors (Kim et al., 2021; Kim and Kim, 2020).
The presence of animal manure odors is a significant challenge in modern industrial society. The concentration of animals in confined spaces, such as feedlots and intensive farming operations, easily leads to the accumulation of odorous compounds (Maurer et al., 2017; Kuroda et al., 2023). These compounds are primarily derived from animal waste and can include ammonia, hydrogen sulfide, methyl mercaptan, and various volatile organic compounds (VOCs) (Kuroda et al., 2022; Lee et al., 2024). Of these, ammonia in particular is a colorless gas with a strong, pungent odor and is one of the designated odorants under the Odor Control Act (Lee et al., 2024).
The various odor abatement methods can be classified into three main categories: physical, chemical, and biological. Among these methods, the use of SCGs as adsorbents for odor removal offers significant environmental and economic benefits (Choi et al., 2022). SCGs have a distinctive porosity that makes them effective at capturing and adsorbing odorants. The use of SCGs is an effective means of eliminating odorous substances that are generated in livestock environments.
In the case of SCGs, they can act not only as adsorbents for contaminant removal, but also as natural carriers for microorganisms. The use of carriers in pollutant adsorption processes is commonly used in bioremediation and has the advantages of being fast, simple, environmentally friendly and cost effective (Dzionek et al., 2016). Prior research has demonstrated that the utilization of carriers for the immobilization of bacteria in microbial decontamination procedures confers the benefit of enhancing the accessibility of contaminants, such as phenol, chromium, DDT, and oil, to microorganisms when adsorbed onto the carrier surface, as compared to their accessibility in the unimmobilized state (Santacruz et al., 2005; Ahmad et al., 2015; Carabajal et al., 2016).
Furthermore, the high nutrient content of SCGs facilitates the activation of microorganisms, which is crucial for odor removal and improvement of the livestock environment in an environmentally sustainable manner. The utilization of a biological approach that employs microbial activity to degrade odor compounds is particularly noteworthy for its contribution to sustainable agriculture (Kim et al., 2021). Previous studies have demonstrated the efficacy of biofertilizers based on beneficial microorganisms (Saha et al., 2023).
Nevertheless, the effective control of odors in livestock farms necessitates not only the implementation of environmental improvements but also the active participation of livestock facility managers in odor management programs. The participation of smaller-scale farms frequently in such initiatives is often constrained, and there is a pervasive lack of awareness regarding the adoption of odor control technology.
This research aims to address the issue of odor generation in livestock facilities by analyzing the potential application of microbial resources and coffee by-products. A further aim of this study to activate and promote the practical application of these resources through experimental trials conducted in the livestock farms of Sunchang. The approach suggested in this study is a promising solution to odor-related challenges in the livestock industry.
Materials & Methods
1. Materials
The coffee grounds (SCGs) were generously provided by a local coffee industry (Redone, Sunchang, Korea). The particles were observed to have a diameter above 0.5 mm, with 90% of the particles exhibiting this characteristic. Additionally, cattle manure was provided by a local cattle farm (Sunchang, Korea). In previous study, Stenotrophomonas rhizophila SRCM 116907 was isolated from paddy soil in order to apply in composting process of sewage sludge (Kim et al., 2023). To identify the microorganisms, 16S rRNA gene analysis was performed by Macrogen Inc. (Seoul, South Korea) using the universal primers 27F (5'-AGAGTTTGATCATGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3'). The partial 16S rRNA sequence of the S. rhizophila strain SRCM 116907 (GenBank accession number OP710251) has been deposited in the GenBank database.
The ammonia nitrogen (NH3-N) and sodium sulfide (Na2S) were procured from Sigma-Aldrich (St. Louis, MO, USA) as the target adsorbate. All chemicals of analytical grade (AR) were utilized in the adsorption experiments without any additional purification. The change in concentration of NH3-N and Na2S in aqueous solution by S. rhizophila SRCM 116907 and SCGs was determined spectrophotometrically (Tecan Spark 10 M, Tecan Trading AG, Switzerland) at 630 nm and 667 nm, respectively (Sugahara et al., 2016; Jain et al., 2021).
2. Analytical methods
The physico-chemical properties of cattle manure samples mixed with SCGs and S. rhizophila SRCM 116907 were assessed, including pH, electrical conductivity (EC), organic matter (OM), ammonia (NH3), nitrate (NO3), hydrogen sulfide (H2S), and mercaptan (Li et al., 2015). 5 g of cattle manure are soaked in 50 mL of deionized water with SCGs (10-40 wt%) and the S. rhizophila SRCM 116907 strain (5 wt%) culture. Subsequently, the mixture is agitated for one hour at room temperature in preparation for further experimentation.
The pH and EC were determined at a 1:5 ratio (manure sample:water, wt/wt) utilizing a pH meter (SevenEasy pH S20, Mettler Toledo) and an EC meter (Orion 3-Star, Thermo Scientific, USA), respectively, in accordance with the established soil test method (Cho et al., 2021). The organic matter (OM) content was determined using the Tyurin method (Šimanský et al., 2019). The concentration of NH3, NO3, H2S, and mercaptan in cattle manure samples was quantified using a Gastec gas sampling pump (GV-100S; Gastec Co., Kanagawa, Japan) (Park et al., 2016).
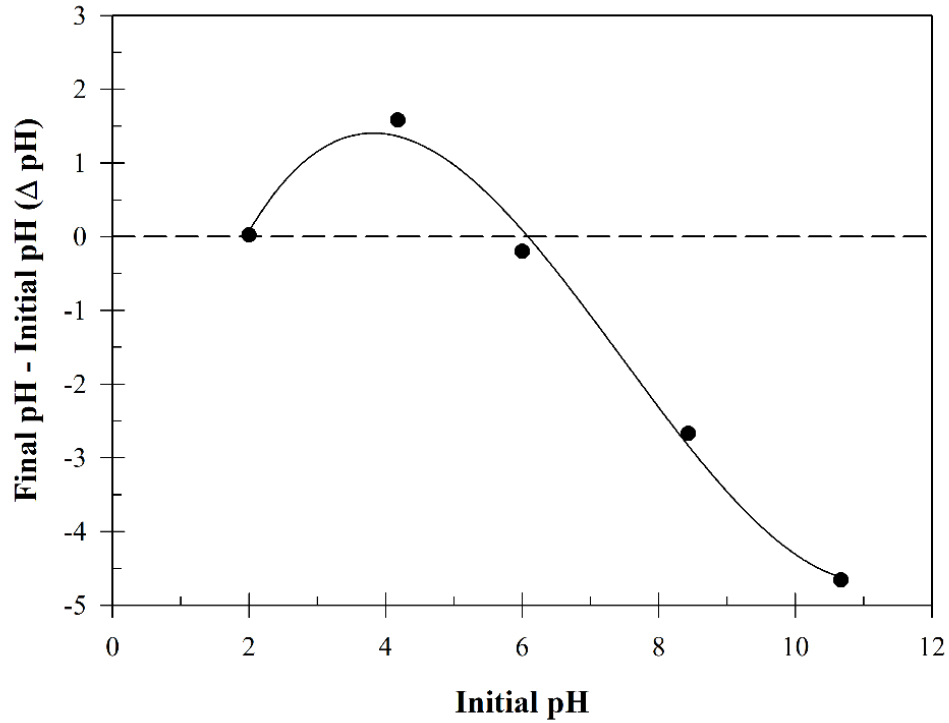
The SCGs were freeze-dried at -80°C using a freeze-drying equipment (FD8508, Ilshinbiobase Co., Ltd, Korea). Fourier transform infrared (FT-IR) spectroscopy (Perkin–Elmer Spectrum 400, PerkinElmer, Inc., Shelton, CT) was employed to verify the presence of functional groups on the surface of SCGs within the 400–4000 cm-1 range. The point of zero charge (pHpzc, ΔpH=0) was characterized by stirring 0.1 g of SCGs in 25 mL (0.1 M NaCl) solution for 2 days (Brouers and Al-Musawi, 2015). The initial pH of the solution was adjusted at 2.0, 4.2, 6.0, 8.4, and 10.7. Delta pH values were calculated as the final pH (pHf)—initial pH (pH0).
3. Adsorption studies of NH3-N and Na2S using SCGs
A 10 mL aqueous solution was prepared with an initial concentration of NH3-N set at 156.5 mg/L, ensuring consistency in the experimental conditions. A range of quantities (0.01 to 0.09 g) of SCGs were added to the 10 mL NH3-N solution in order to investigate the adsorption capabilities of SCGs. The varying quantities of SCGs permitted a comprehensive investigation of the dose-dependent relationship.
A 30 mL aqueous solution was prepared with an initial concentration of Na2S set at 52.21 mg/L, which was selected to simulate conditions relevant to water treatment processes. A series of additions of coffee grounds (0.01 to 0.09 g) were made to the 30 mL Na2S solution in order to assess their impact on the removal efficiency of Na2S. The adsorption capacity (qe) and removal efficiency of NH3-N and Na2S in aqueous solution were expressed as follows:
Adsorption capacity:
Removal efficiency (%) =
Where C0 represents the initial concentration (mg/L), Ct represents the final concentration (mg/L), V stands for the volume of the reaction (L), and W denotes the weight of SCGs (g).
4. Adsorption kinetic and isotherm models of NH3-N and Na2S using SCGs
In order to investigate the NH3-N and Na2S adsorption mechanism and adsorption efficiency on the surface of SCGs as natural carriers for the immobilization of bacteria, a series of kinetic and isotherm experiments were conducted, respectively. The adsorption rate and mechanism were investigated through the application of the pseudo-first-order and pseudo-second-order models. A series of kinetic experiments were conducted utilizing 0.03 g of SCGs, an initial concentration of NH3-N (165.3 mg/L) and Na2S (28.52 mg/L) solution. The non-linear forms of the adsorption kinetic (Pseudo-first-order and Pseudo-second-order) and isotherm (Langmuir and Freundlich) models were expressed as follows:
Pseudo-first-order model:
Pseudo-second-order model:
Langmuir isotherm model:
Freundlich isotherm model:
Where qt and qe (mg/g) represent the amount of absorbents at time (min) and equilibrium; k1 and k2 are the kinetic rate constants for the non-linear pseudo-first-order (min-1) and pseudo-second-order (g/mg/min) models; kp represents the linear form of the intra-particle diffusion constant (mg/g/min0.5), and C is the intercept.
5. DNA extraction and next generation sequencing (NGS)
DNA was extracted from cattle manure, SCGs containing manure, and bacteria-inoculated manure using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. The purity and quantity of the extracted DNA were assessed using the NanoDrop spectrophotometer (Thermo Fisher Scientific, 5225 Verona Rd, USA) and the Invitrogen Qubit 4 Fluorometer (Invitrogen Inc., Carlsbad, CA, USA), respectively.
To profile the microbial communities, we constructed amplicon libraries targeting the hypervariable V3-V4 region of the 16S rRNA gene. The 341F (5’-CCTACGGGNGGCWGCAG-3’) and 785R (5’-GACTACHVGGGTATCTAATCC-3’) primers were employed for this purpose, as previously described by Pacwa-Płociniczak et al. (2020). The library was prepared using the Nextera XT Index Kit v2 (Illumina, REF 15052163), and the sequencing was conducted on the Illumina MiSeq platform (Illumina Inc., San Diego, CA).
Results & Discussion
1. Influence of SCGs in cattle manure properties
In order to identify the primary causes of odor in livestock buildings and to develop an effective odor control process, we conducted a study to examine the changes in characteristics based on the composition of cattle manure, SCGs, and S. rhizophila SRCM 116907 mixed manure. The results of the analyses are presented in Table 1, which summarizes the variations in properties with different proportions of cattle manure and SCGs in the mix.
Table 1.
Chemical properties of the experiment cattle manure, SCGs, and S. rhizophila SRCM 116907 mixed samples.
| Samples |
pH (1:5) | EC (dS/m) | OM (%) |
NH3-N (mg/L) |
NO3-N (mg/L) |
H2S (mg/L) |
Mercaptan (mg/L) |
| Cattle manure | 7.11±0.04 | 1.75±0.01 | 63.06±1.5 | 202.15±5.48 | ND | 60.3±2.3 | ND |
| SCGs | 5.66±0.02 | 4.52±0.56 | 83.37±0.56 | ND | ND | ND | ND |
| Manure + SCGs (10%) | 6.98±0.06 | 1.90±0.07 | 67.65±2.98 | 83.91±14.20 | ND | 44.2±1.6 | ND |
| Manure + SCGs (20%) | 6.75±0.01 | 2.20±0.02 | 75.45±0.36 | 58.58±5.18 | ND | 19.2±1.3 | ND |
| Manure + SCGs (40%) | 6.59±0.01 | 3.48±0.03 | 78.6±0.61 | 46.98±1.83 | ND | 9.3±0.8 | ND |
| Manure + SCGs (10%) + SRCM 116907 | 6.81±0.08 | 2.01±0.04 | 70.65±1.04 | 63.18±4.46 | ND | 30.4±1.2 | ND |
The variations in pH, electroconductivity (EC), organic matter (OM), ammonia-nitrogen (NH3-N), nitrate-nitrogen (NO3-N), hydrogen sulfide (H2S), and mercaptans with different mixtures provide valuable insights into the potential impact of the combination of cattle manure, solid cattle manure granules (SCGs), and S. rhizophila SRCM 116907 on the characteristics of the cattle manure. Further discussion will examine the implications of these variations in the context of odor reduction strategies and the development of efficient odor control processes for livestock facilities.
A range of SCGs, with concentrations varying from 10 to 40% (wt/wt), were added to cattle manure. The pH of the SCGs was found to be 5.66, which is a relatively lower value compared to that of the cattle manure (pH 7.11). Moreover, a discernible decline in pH was observed as the concentration of SCGs in the cattle manure mixture increased (Orfanoudaki et al., 2020). The observed variation in pH is a significant finding, as it reflects changes in the acidity or alkalinity of the manure when SCGs are incorporated. The relatively lower pH of SCGs suggests the potential for acidity contributions to the mixture. As the concentration of SCGs in cattle manure increases, the marked decrease in pH indicates that the environment becomes more acidic.
An increasing trend in electrical conductivity (EC), indicative of elevated conductivity, was observed in cattle manure mixtures with increasing SCGs content. This is consistent with the findings of Zhang et al. (2021), who reported that coffee by-products, including SCGs, are rich in carbohydrates, proteins, minerals, significant amounts of polyphenols, and caffeine. The observed increase in EC may be attributed to the substantial presence of polyphenols and caffeine in SCGs.
The presence of NH3-N and H2S, both of which are known to be significant contributors to odor in livestock waste, was observed at relatively high concentrations of 202.15 and 60.3 mg/L, respectively, in the cattle manure sample. However, the concentration of NO3 and mercaptan was not detected in either the cattle manure or the SCGs sample.
A negative correlation was observed between the concentration of NH3-N and the content of SCGs in the mixture of cattle manure and SCGs. As the concentration of coffee grounds increased, a significant reduction in NH3-N was observed. In particular, the mixture was treated with 40% (wt/wt) SCGs, which resulted in a maximum reduction effect of 76.76%. The application of 10 wt% SCGs in a mixture with S. rhizophila SRCM 116907 to cattle manure resulted in a notable reduction in the levels of NH3-N and H2S, with a reduction of approximately 68.7% and 49.6%, respectively.
2. Analysis of efficiency of removing odor-causing substances (NH3-N and Na2S) from SCGs
Due to the rapid atmospheric oxidation of hydrogen sulfide (H2S) over time, conducting waterborne adsorption experiments using SCGs is challenging. This study employed sodium sulfide nonahydrate (Na2S·9H2O), which is water-soluble, as a model substance for H2S. The use of Na2S·9H2O permitted the accurate and controlled measurement of hydrogen sulfide concentrations within the experimental setup (Sugahara et al., 2016). Na2S and NaHS has been employed extensively as a H2S donor. Upon dissolution in water, Na2S readily decomposes, releasing H2S. Equation 1 shows that Na2S (water soluble) is formed by the reaction between H2S and NaOH (Shahrak et al., 2015).
A series of removal efficiency analysis experiments were conducted to evaluate the effect of different SCGs dosages (0.01~0.09 g) on the removal efficiency of NH3-N and Na2S. The results of these experiments are presented in Figure 1, which provides insight into the effectiveness of SCGs in reducing NH3-N and Na2S concentrations in the livestock waste environment.
The study of the removal of NH3-N in aqueous solution based on different doses of SCGs yielded significant results. The maximum adsorption capacity was observed when 0.09 g of SCGs was added, reaching 39.2 mg/g (Figure 1A). Furthermore, the maximum removal efficiency was observed when 0.09 g of SCGs was added, resulting in a remarkable 25.1% reduction in NH3-N within 12 h.
The adsorption capacity of Na2S was investigated with varying amounts of SCGs introduced. The results demonstrated that the maximum adsorption occurred at 0.01 g, reaching 46.49 mg/g (Figure 1B). Moreover, a consistent trend of decreasing adsorption capacity was observed as the amount of SCGs increased. Specifically, at 0.09 g of SCGs, the removal rate reached a maximum of 79.2%. This result indicates that the increased presence of SCGs in alkalized aqueous solution by Na2S led to the aggregation of the adsorbent during the adsorption of sodium sulfide, possibly due to a screening effect. Furthermore, the adsorption capacity is also reduced due to the agglomeration of SCGs, which reduces the total surface area of the adsorbent (Demir et al., 2019).
3. Adsorption kinetics and isotherms of NH3-N and Na2S using SCGs
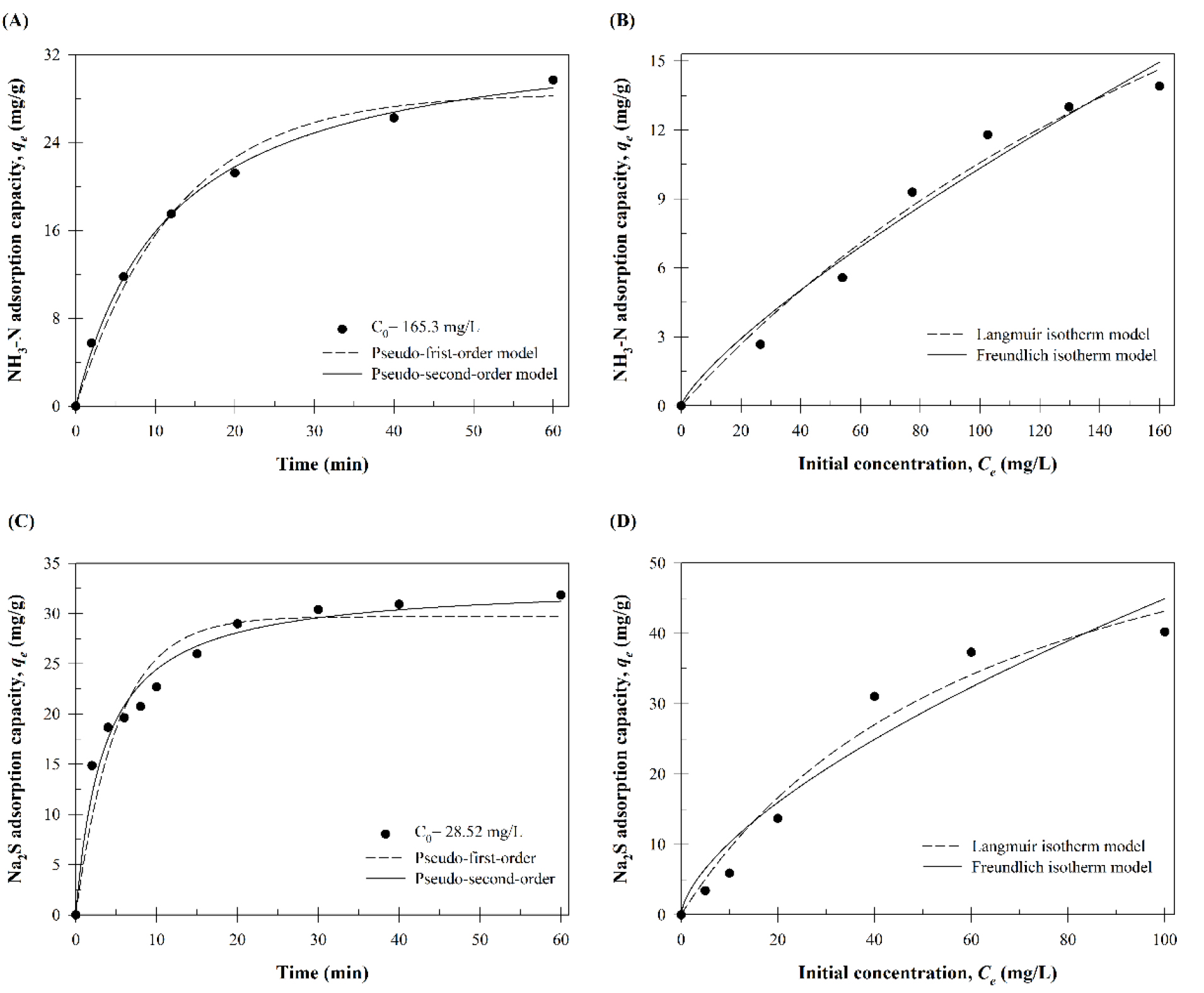
A kinetic analysis was conducted to evaluate the changes in NH3-N and Na2S adsorption amounts over time intervals (Figure 2A, C). Furthermore, the alterations in the adsorption capacity of SCGs were examined in relation to different initial concentrations. The results of the analysis were employed to apply the isothermal adsorption model (Figure 2B, D). Table 2 presents the kinetic and isotherm model parameters for the adsorption of NH3-N and Na2S by SCGs.
Table 2.
NH3-N and Na2S adsorption kinetic and isothermal adsorption model parameters measurement results.
Following analysis, it was determined that NH3-N achieved adsorption equilibrium within approximately 60 minutes, with a confirmed maximum adsorption amount (qexp) of 29.68 mg/g. Similarly, Na2S reached adsorption equilibrium in approximately 30 minutes, with a maximum adsorption amount (qexp) of 31.83 mg/g (Table 2). Upon substitution of the NH3-N and Na2S adsorption data into the pseudo-first-order and pseudo-second-order models, it was found that the coefficient of determination (R2) was higher for the pseudo-second-order model. Furthermore, the Langmuir and Freundlich isotherm models were employed, and it was established that the Langmuir model yielded a suitable R2 for the adsorption of NH3-N using SCGs.
The results indicate that the adsorption of NH3-N by SCGs is predominantly driven by chemical adsorption or ion exchange mechanisms, rather than physical adsorption (Vazquez et al., 2002). The theoretical maximum adsorption capacity for NH3-N was calculated to be 40.89 mg/g, while that for Na2S was found to be 71.86 mg/g, indicating a monolayer adsorption tendency for both substances.
4. Characterization of SCGs: pH and FT-IR analysis
The pHpzc (point of zero charge) of an adsorbent is the pH at which the net charge of a given surface or particle becomes neutral (Rosson et al., 2020). This value represents the point at which the surface of a material undergoes a transition from a positive to a negative charge, a phenomenon that is often associated with adsorbents or catalysts. To ascertain the relative surface potential of the SCGs, pHpzc analysis was conducted using 0.1 M NaCl, and the results are presented in Figure 3. Zeta potential analysis was conducted over the pH range of 2.0 to 10.7, and the pHpzc of the SCGs was determined to be 6.1. These results indicate that below pH 6.1, the surface of the coffee grounds is predominantly positively charged, while at pH 6.1, a shift to a dominant negative charge is observed.
In the context of cattle manure, pH variations are influenced by a number of factors, including season and animal species. The pH level observed in this study for cattle manure was approximately 7.1. Upon the addition of 40% (wt/wt) SCGs, the pH decreased to approximately 6.59. These findings suggest the potential for an increase in the ratio of negative charges on the surface potential of SCGs in cattle manure samples.
The surface functional groups of SCGs were characterized using FT-IR analysis, and the results are presented in Table 3. The identification of the functional groups on the surface of the adsorbent is of critical importance in order to gain insight into the adsorption mechanism (Pellenz et al., 2023). The Table 3 provides a detailed account of the identified functional groups and offers insights into potential mechanisms that may influence the adsorption of NH3-N and Na2S.
Table 3.
Confirmation of ammonia nitrogen and sodium sulfide removal mechanism based on IR spectra.
| Functional groups (Wavenumber) | Adsorption mechanisms | References |
| -OH or -NH stretching (3325.75 cm-1) | -OH···NH3+ or R-NH3+···Na2S | Ning et al., 2018 |
| C-H stretching (2923 and 2855 cm-1) | - | - |
| C=O (1742.79 cm-1) | - | - |
| Diketones (1646.96 cm-1) | - | - |
| Carboxyl, -COOH (1454.77 cm-1) | -COOH···NH3+ | Zhu et al., 2020 |
| Ester carbonyl, RCOOR' (1155.24 cm-1) | -RCOOR'···NH3+ | Kim and Park, 2007 |
| Alkyl amine (1030.67 cm-1) | - | - |
| C-Cl (713.04 cm-1) | - | - |
The FT-IR spectra exhibit a series of prominent bands that correspond to the -OH stretching vibration (or N-H stretching of the amine) at approximately 3325 cm-1. Furthermore, the peaks observed at 1454 cm-1 and 1155 cm-1 are attributed to the carbonyl (-COOH) and ester carbonyl (RCOOR'), respectively. The mechanism of NH3-N and H2S adsorption by these functional groups has been previously reported in the literature (Kim and Park, 2007; Ning et al., 2018; Zhu et al., 2020).
5. Inoculation of a microorganism for the reduction of the odor of cattle manure
In a previous investigation by Kim et al. (2023), the S. rhizophila SRCM 116907, which is characterized by its diverse enzyme activities, including protease and cellulase, demonstrated a remarkable NH3-N removal efficiency of 24.2% and exhibited positive effects on plant growth, as evidenced by the growth of Lolium perenne and Chrysanthemum burbankii. In the study, the strain was used to inoculate SCGs on cattle manure. The impact of the combined treatment on the microbial community was validated through next-generation sequencing (NGS) analysis.
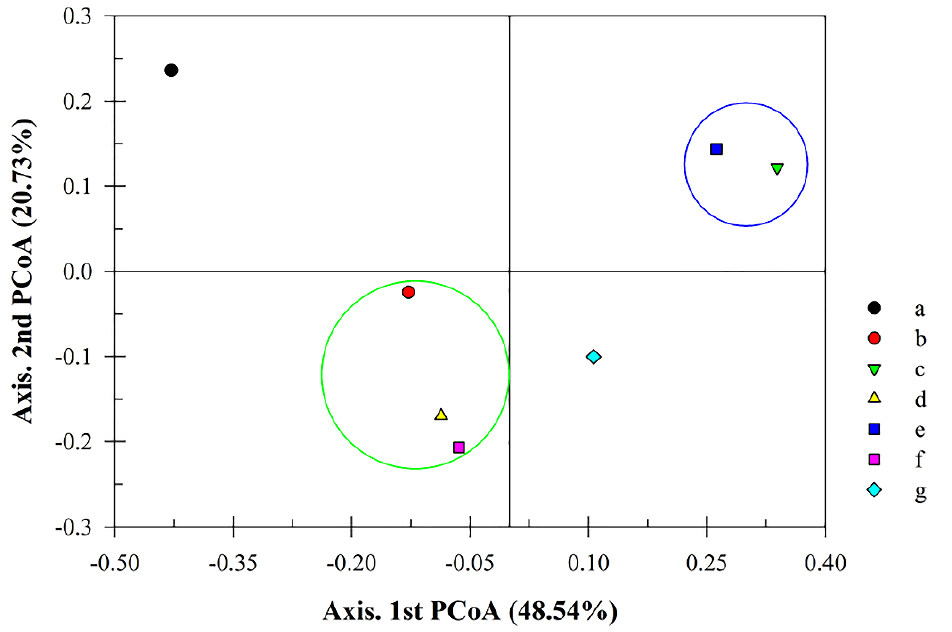
The objective of this study was to assess the impact of inoculating a S. rhizophila SRCM 116907 strain with the aim of mitigating the odor of cattle manure on the microbial community. To this end, next-generation sequencing (NGS) analysis was employed. The results of the alpha-diversity analysis are presented in Table 4. The experiment tested various conditions, including a control group using cow manure, a group using 40% SCGs without microorganism treatment (SCG W/O B), a group using 40% SCGs with microorganism treatment (SCG W/B), a group without microorganism treatment on the first day (W/O B 1 day), a group with microorganism treatment on the first day (W/B 1 day), a group without microorganism treatment on the third day (W/O B 3 days), and a group with microorganism treatment on the third day (W/B 3 days).
Table 4.
Microbial community changes (alpha-diversity) according to cattle manure application.
The Operational Taxonomic Units (OTUs) and Chao indices, which indicate the abundance of microorganisms in the samples, exhibited the highest values in the raw material. Furthermore, the uninoculated group exhibited higher values than the treated group that was inoculated with microorganisms. Furthermore, the uninoculated group exhibited a higher microbial abundance than the SCG-treated group. The Shannon index, which reflects microbial diversity, exhibited a consistent trend in parallel with the abundance indices.
The analysis of alpha-diversity revealed that treatments involving SCG, microbial inoculation, and aerobic treatment led to a reduction in both microbial diversity and abundance in cattle manure. To confirm the correlation between the microbial communities in each sample, their similarity was evaluated using Bray-Curtis dissimilarity in principal coordinates analysis (PCoA) (Figure 4). The analysis demonstrated significant differences between the control group and groups B, D, and F, which were not treated with microorganisms, as well as groups C and E, which were treated with microorganisms and cultured for one day.
Conclusion
In conclusion, this study investigated the potential of SCGs as a means of reducing odor in cattle manure. The addition of SCGs resulted in significant alterations in pH, EC, NH3-N and H2S, thereby indicating their potential for odor reduction. Specifically, SCGs demonstrated a substantial reduction in NH3-N and Na2S at a 40 wt% concentration, with a 76.8% reduction in NH3-N and an 84.6% reduction in Na2S. Furthermore, Treatment with 10 wt% SCGs + S. rhizophila SRCM 116907 in cattle manure resulted in a significant reduction of approximately 68.7% in NH3-N and 49.6% in H2S, respectively. Adsorption analyses of NH3-N and Na2S using SCGs in aqueous solution indicated that chemical or ion exchange mechanisms were likely involved, with monolayer adsorption tendencies being a key factor. The dynamic surface potential and functional groups of SCGs were characterized, providing insight into the adsorption mechanisms. The inoculation of S. rhizophila SRCM 116907 demonstrated positive effects on the removal of NH3-N and Na2S, as well as on the structure of the microbial community. This represents a potential integrated strategy for reducing odors, which could be a valuable addition to the existing arsenal of odor control techniques. The results of this study demonstrate the potential of SCGs in enhancing manure properties and microbial dynamics, thereby contributing to sustainable odor control in livestock facilities. Nevertheless, further discussion is required to ascertain the most effective methodology for improving adsorption efficacy, taking into account variations in external environmental factors such as temperature, the presence of interfering ions, and the frequency of recycling.